FDA Approval of Pfizer-BioNTech Vaccine Lessens Public Weariness
September 3, 2021
2020. The year is easily recognized as the year of COVID-19 and quarantine. The year when students lived in their bedrooms and business owners scrambled to find ways to keep their doors open. Now, in 2021, vaccines have been created and restrictions have been lifted; the US is ready to open back up.
The FDA approval of the Pfizer COVID-19 vaccine is a big step in this process.
The vaccine was previously available under Emergency Use Authorization, which meant it could be used in public health emergencies as long as the benefits outweighed the risks. FDA approval, however, is a much more complex process of evaluating the safety, quality and effectiveness of products such as the Pfizer vaccine.
The FDA guaranteed that, although the approval was expedient, they were still thorough, analyzing thousands of pages of data. They are hoping to make unvaccinated people more confident in the safety of the vaccine.
Commissioner Janet Woodcock said, “While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated.”
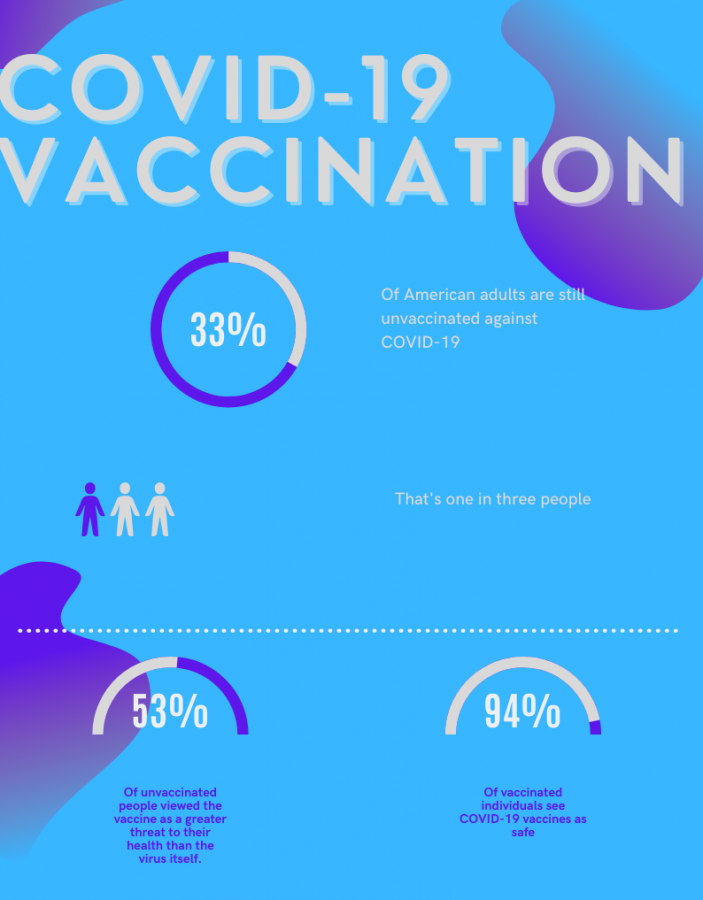
A Kaiser Family Foundation survey revealed that 53 percent of unvaccinated adults in July hadn’t received a COVID-19 vaccine because they viewed the vaccine itself as a greater threat to their health than the virus itself. Only seven percent of vaccinated individuals feel this way.
The majority of vaccinated people view getting COVID-19 as a greater risk than the risk of receiving the vaccine. One of these individuals is senior Katlyn Finkel, who was confident in the vaccine’s safety after the initial EUA.
“There’s no reason to believe that it isn’t safe,” she said. “It had been tested and approved by the CDC at that point and was the only way for our lives to get back to normal.” Finkel said that, due to this, the FDA approval had no significant meaning to her.
However, with 33 percent of adults unvaccinated as of July, the FDA hopes to help unvaccinated individuals feel more comfortable getting the vaccine through the approval.